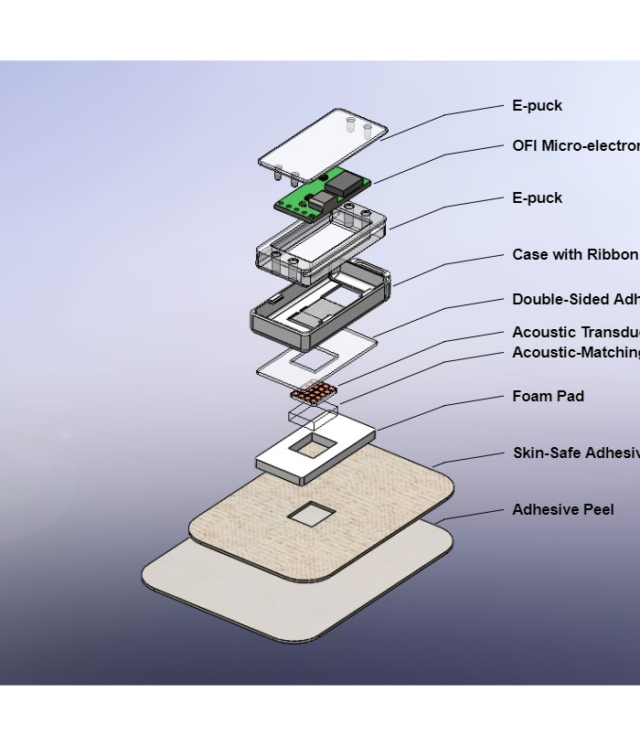
Orthoforge has created an adjunct technology to the current standard of care for fractures. Their solution is disruptive (better outcomes at substantially lower costs) and will change the standard of care for countries worldwide.
This solution will provide the clinician with quantifiable/actionable data regarding the patient’s specific fracture healing progress with no additional harmful X-ray ionizing radiation. It measures the fracture with approximately 2000 data points daily and provides the clinician with a dashboard (that can be seen below) that presents comprehensive healing and trending data of the healing process that improves outcomes. This solution is the next step in increasing patient quality of care, improving outcomes, and bringing fracture healing into the 21st century. All these benefits for the patient are achieved along with tremendous savings for the payee: making the process highly disruptive.
It does all this with an average savings of ~$9K USD per fracture, no additional doctor visits, and reduces loss of productivity (wages, school, time) for the patient or their family. Orthoforge enjoys a profit of 75% on hardware and 99% on software.
This opportunity is eligible for RRSP, RESP and TFSA accounts. For more information and to book a complete presentation, don’t hesitate to contact us at paul@npn.ca.